Digital therapeutics (DTx), unlike traditional treatments such as pills, uses software installed in smartphones or wearable devices as software as a medical device (SaMD) to cure diseases and improve health conditions, which is a major departure from existing wellness products (e.g., Fitbits). As with traditional therapeutics, DTx also requires clinical validation of efficacy through systematic clinical trials. The US FDA has already authorized a number of DTx products, for example, WellDoc's BlueStar for diabetes management, and Pear Therapeutics' reSET for drug addiction recovery, opening up new DTx possibilities, such as doctors' prescriptions and insurance reimbursement. Unlike traditional drug development, the cost of DTx development is relatively low, and new DTx markets are growing rapidly. The DTx market is estimated to increase to $8.7 billion in 2025, with an average annual growth rate of 20% (GrandView 2017).
DTx therapies mostly consider behavioral changes in chronic diseases (e.g., diabetes and cardiovascular diseases) and neuropsychiatric diseases (e.g., depression, sleep disorders, and ADHD). These are the areas in which the treatment effects of cognitive behavior therapies are significant. DTx therapies can deliver patient-centered care by supplementing the areas in which treatment is difficult or poorly managed through existing treatment methods (e.g., lifestyle coaching and cognitive behavior therapies), to improve the quality of care at lower costs.
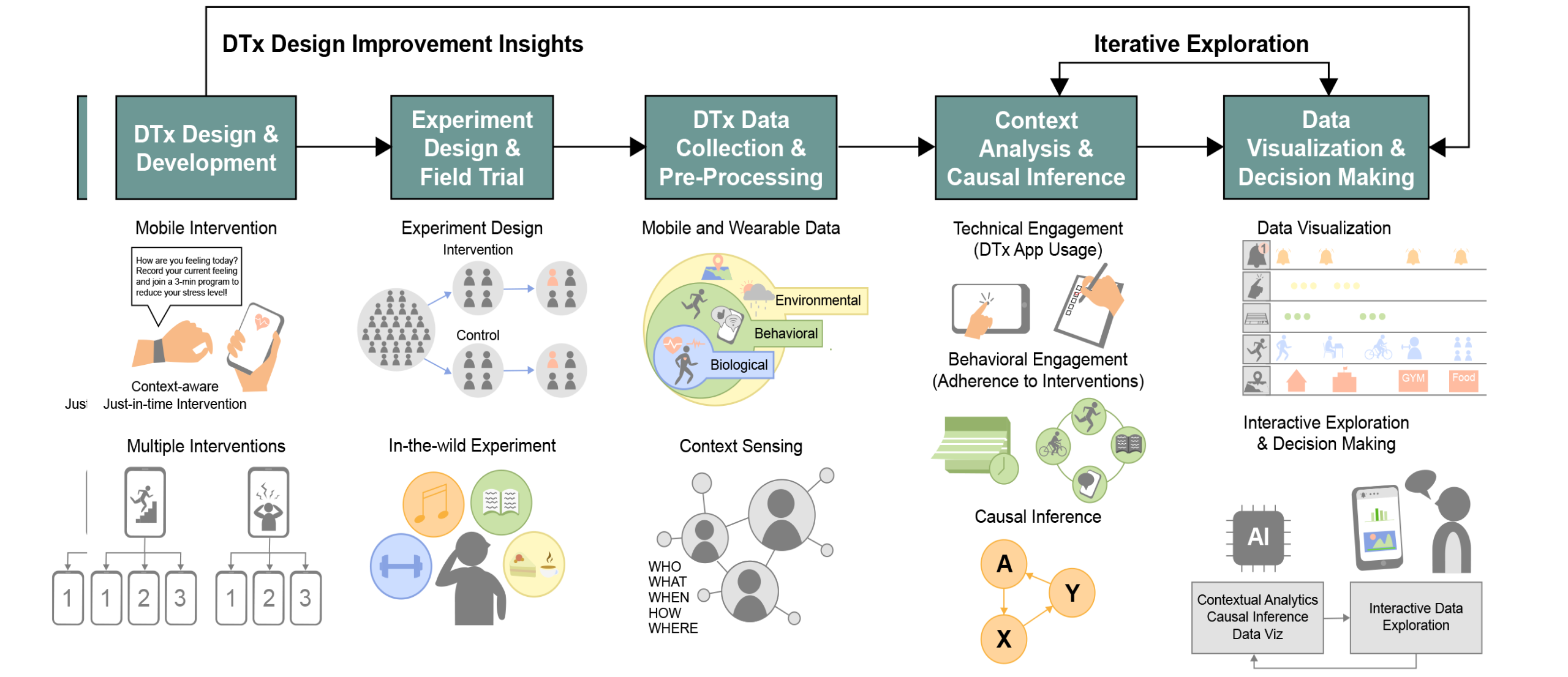
One of the important components of traditional drug development is the selection and optimization of a drug delivery system that aims to effectively deliver a specific drug to the desired target (e.g., sustained release with microneedle patches). Digital therapeutics can deliver various interventions through digital technologies (e.g., interactive mobile content, videos, chatbots, and push notifications). Thus, it is very important to analyze and optimize the engagement and receptivity of “DTx delivery systems”' using mobile and wearable devices.
The receptivity to DTx relates to the overall process of intervention delivery using digital devices (e.g., notification delivery, notification perception/checking, and behavioral adherence). DTx aims to induce behavioral changes in users, and it is very important to analyze patient DTx engagement and receptivity to shorten the DTx development time and to maximize the effectiveness of DTx. This project aims to investigate the user engagement and intervention receptivity to DTx by analyzing digital footprint data (known as digital phenotype data) collected from mobile and wearable devices. Data-driven DTx analytics for user engagement and intervention receptivity in DTx delivery systems will provide key insights for the improvements of DTx, possibly innovating the existing paradigm of DTx development processes.


